Fasciacytes – a newly discovered cell type responsible for fascial sliding:
The composition of the deep fascia is a complex multi-planar arrangement of several layers of closely packed collagen fibres interspersed with some elastic fibres, that is separated by loose connective tissue and bathed in hyaluronic acid (HA) (Benetazzo et al 2011). Many authors have published on the abundant proprioceptive supply within and especially between the layers of this tissue, with some indicating that connective tissue (rather the retina) is the most receptor rich tissue in the human body. In addition to these receptors, the deep fascia is also populated with lymphatic and vascular channels as per Stecco et al 2007 and Bhattacharya et al 2011.
According to two friends and colleagues of mine, Drs. Kumka and Bonar in 2012, the fascia is responsible for numerous functions including force transmission, movement, stability, proprioception and promoting sliding and reducing the friction associated with motion. Both Chaitow 2014 and Langevin et al 2011 report that thickening and densification of the loose connective tissue and its extracellular matrix (ECM) corresponds to the reduction or loss of fascial sliding ability – this has an effect on both the concentric and eccentric portions of movement around a joint. Eccentrically the connective tissue must lengthen in order to allow motion to occur, a principle which many are comfortable understanding. Concentrically, there needs to be proper fascial mobility for a different reason. The tension that propagates through normal healthy connective tissue will stretch alpha motor neurons, and they in turn will active our gamma motor neurons leading to proper muscle contraction. Connective tissue that lacks resilience will not readily trigger this normal mechanism, and as result our motor output is altered. Now also consider the faulty information that will arise from unhealthy, less extensible connective tissue – given that we base, alter and adjust our movement on the proprioceptive information we receive from connective tissue, which is the most receptor-dense tissue we have, you can see how imperative healthy, resilient, extensible and properly sliding connective tissue is to human movement.
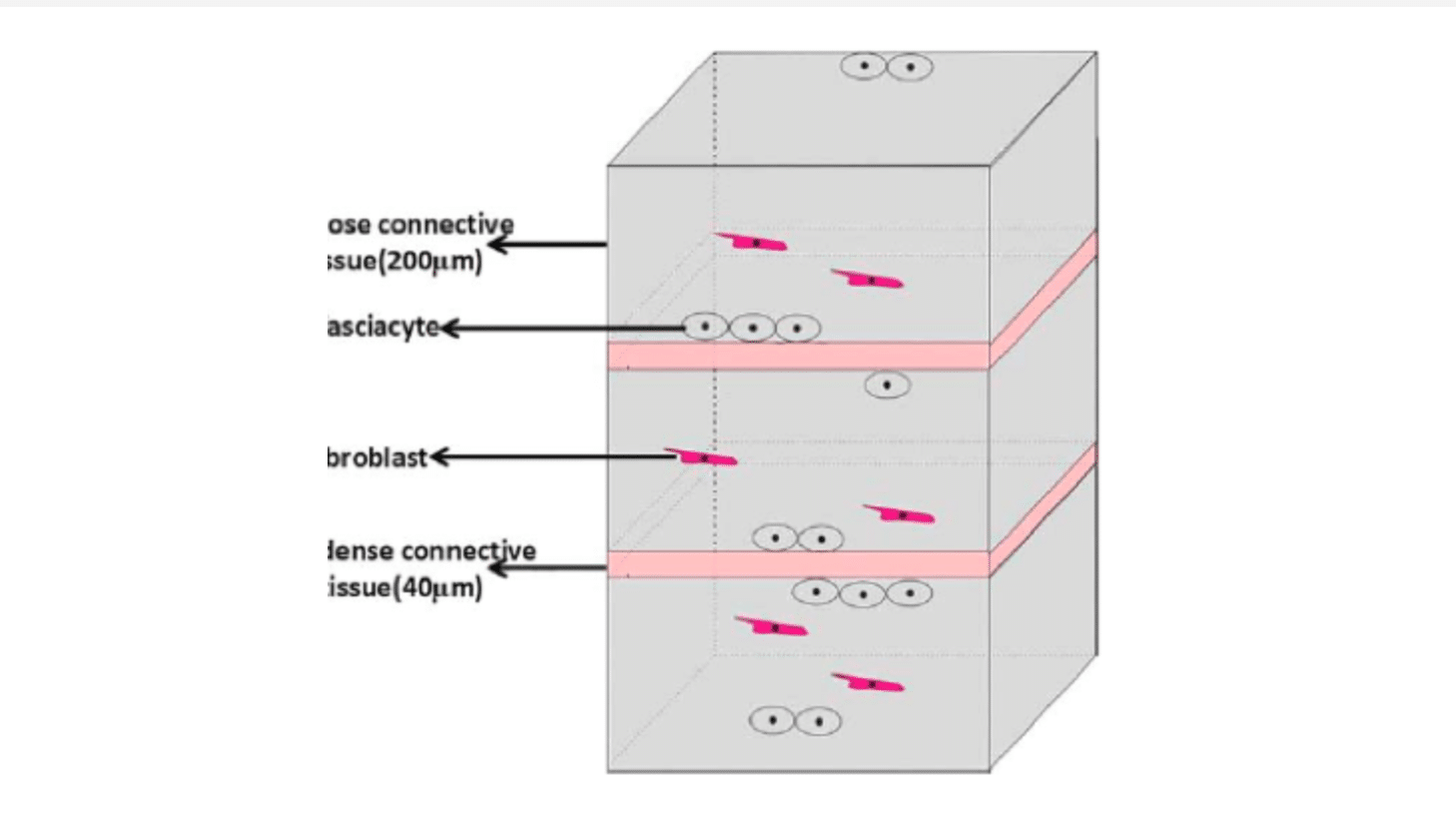
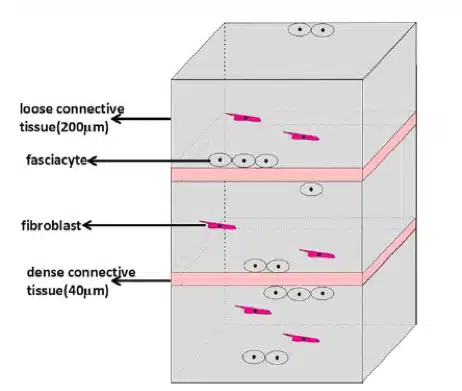
What then are the keys components to mitigating this fascial density? According to Stecco et al 2014, one such element is HA. Synthesized within the plasma membranes of most cell types, HA serves a range of functions including space filling, lubrication of joints, water homeostasis, and provision of a matrix that facilitates cell migration according to Fraser et al 1997 and Cowman et al 2015. More recently, it has also been demonstrated that HA provides a substrate for the smooth gliding of different motor units between and within muscle as noted by McCombe et al 2001. Stecco et al 2001 found fibroblast-like cells on the inferior surface of the deep fascia that were specialized for HA synthesis and secretion, bearing a marked similarity to synoviocytes on the inner surfaces of joint capsules and to hyalocytes in the eye that produce the HA-rich humors of the anterior and posterior chambers. Carla Stecco suggests that these represent a previously-unidentified class of cells that we called “fasciacytes.” Her recent paper in Human Anatomy March 2018 characterized for the first time fibroblast-like cells in fasciae specialized for the biosynthesis of the HA-rich matrix. They are morphologically distinct from classical fibroblasts in that they have rounder nuclei, cytoplasm restricted to the perinuclear region, and smaller and less elongated cellular processes. In addition, while fibroblasts lie among the collagen fibrous bundles in the fibrous fascial sublayers, fasciacytes form small clusters along the surface of each fascial sublayer, defining the boundary between the fibrous sublayer and the loose connective tissue.
The picture below shows the size and shape of fasciacytes – they are the round dark blue dots surrounded by a ring of paler blue.

This pictorial representation of the fasciacytes location, clustered under each fascial sublayer, suggests that regulation of the activity of these cells in HA production could modulate gliding between adjacent fibrous fascial sublayers, which seems to be strongly related to myofascial pain and range of movement as reported by Langevin et al 2011.
What is the clinical significance of these cells? Previous research by Temple-Wong et al 2016 has shown that changes in HA concentration are associated with inflammatory and degenerative arthropathies and that defects in fascial gliding can compromise the entire functionality of tissues and can generate pain according to Bordoni and Zanier 2014. It has yet to be determined what stimuli these new cells respond to or how they respond to it. Given that a lack of sliding correlates with a densification of the loose connective tissues, it is plausible that treatments aimed at the areas where densification occurs and focused on eliminating them will yield and improvement in fascial sliding, and therefore mobility, as a result of better directional information from connective tissue, a lack of eccentric movement restriction, and proper concentric muscle activity. These areas are the Neurodensification Points we use in the Integrated Seminar Series approach, and we have noted immediate positive effects on mobility, movement patterns, and overall symptoms.